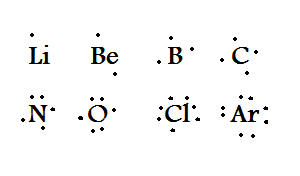Outstanding Tips About How To Draw An Electron Dot Diagram

Mg 2+, s 2−, draw the lewis electron dot diagram for each ion.
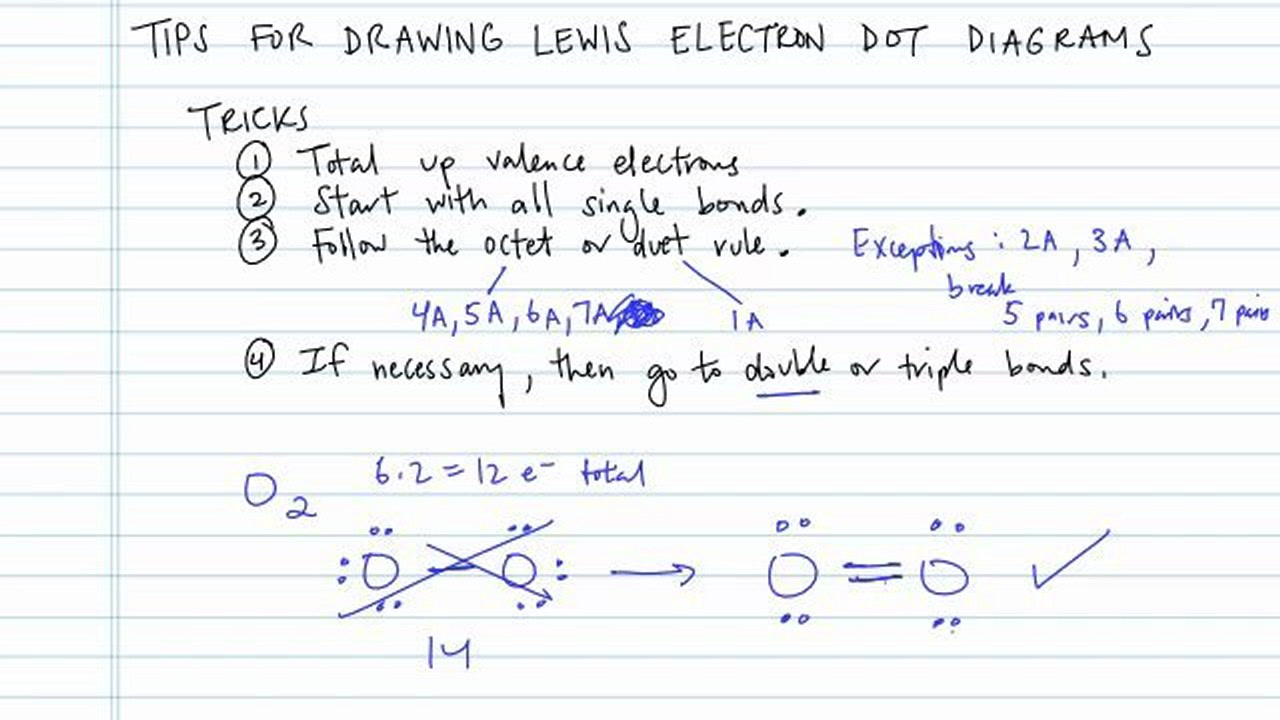
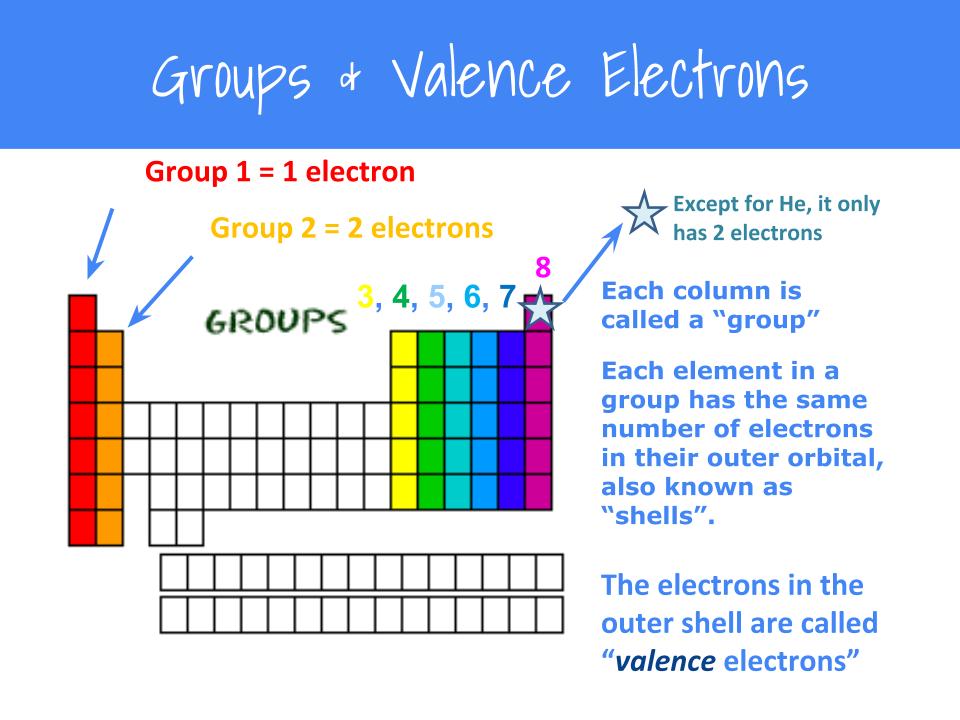
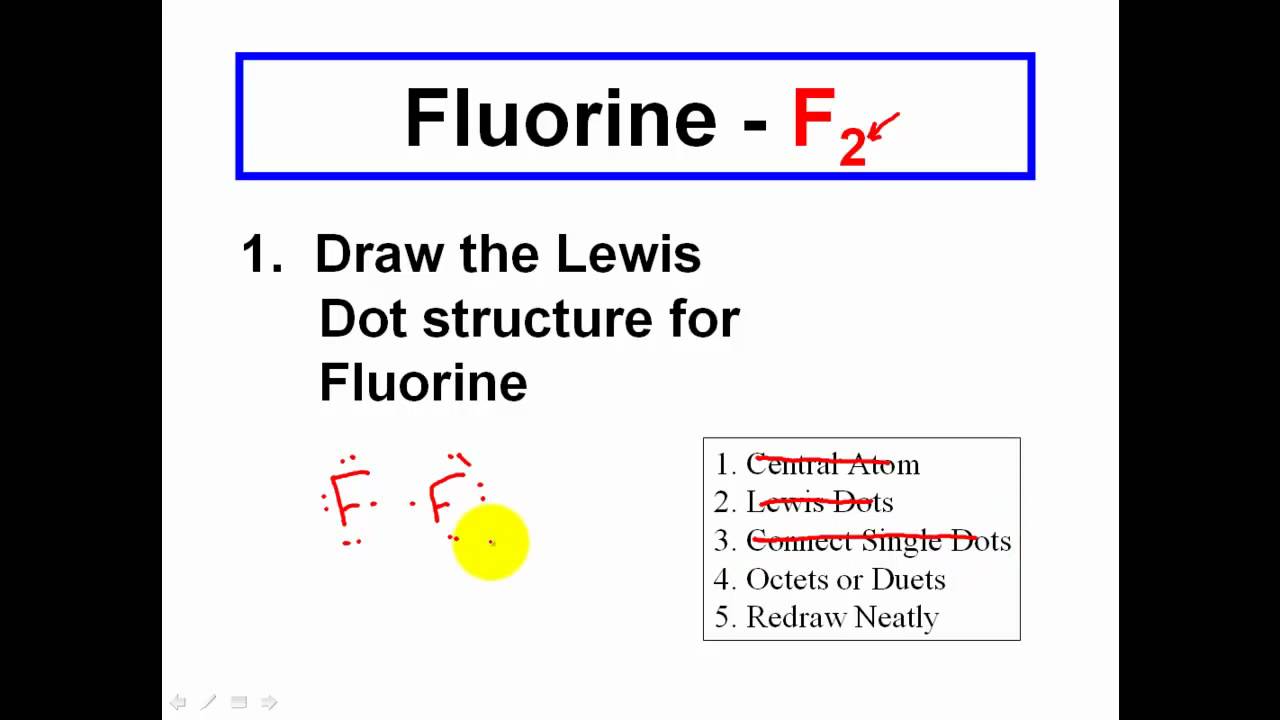
How to draw an electron dot diagram. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Oxygen is in group vi of the periodic. Andersen shows you how to draw lewis dot diagrams for atoms and simple molecules.intro music atributiontitle:
To draw lewis dot diagrams, follow these steps: Draw the lewis electron dot diagram for each ion. Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol.
The atomic number tells you how many electrons to draw in total. Add single covalent bonds to. A beryllium atom, with two valence electrons, would.
With elemental phosphorus (white phosphorus, p4) as a bonus.check me out: Find the total number of valence electrons; How do you draw an electron dot diagram for an element?
How to draw the lewis dot structure for clbr3: Sketch out the electron configuration diagrams for each of the atoms. The steps that must be followed while.
Count the number of valence electrons in the molecule. For example, potassium has 19 electrons, draw a small circle and write the symbol in the centre. Shared pairs of electrons are drawn as lines between atoms,.
Step 2 tells how many. Place one electron pair between. Lewis structures can be made for molecules that.
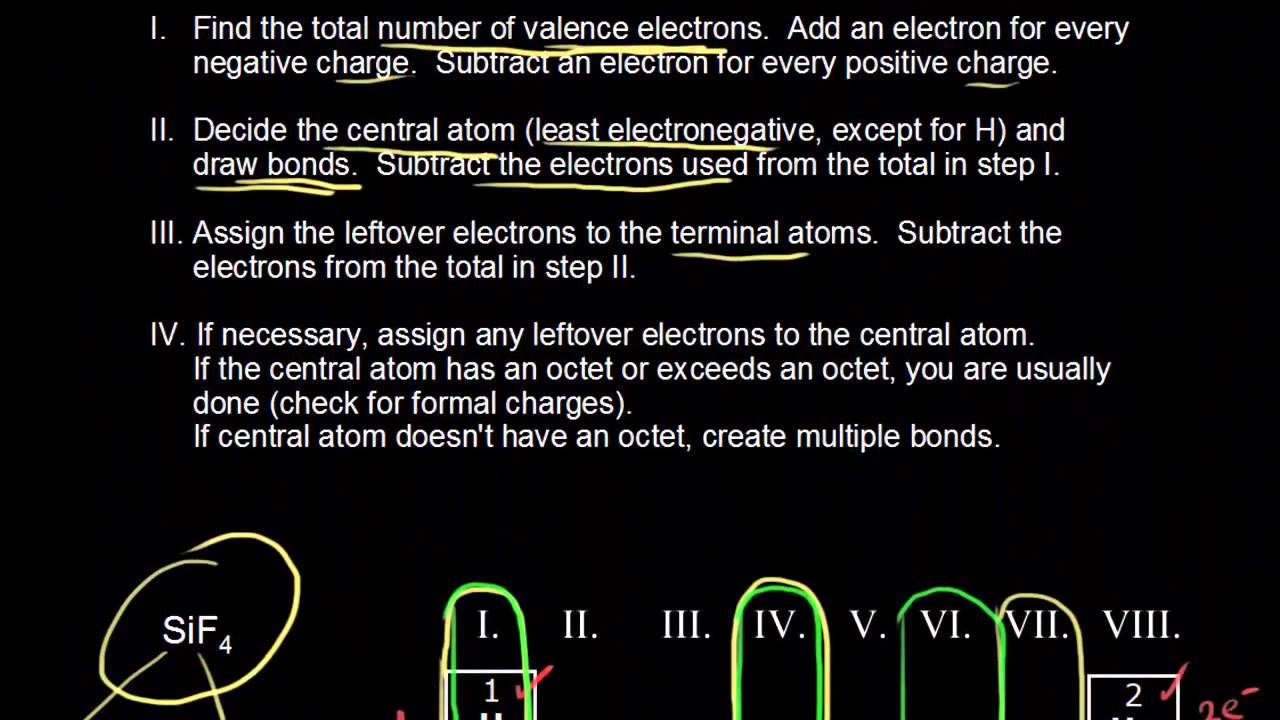
For anions add a number of electrons equal to the negative charge. Write the symbol of the atom you are drawing the electron dot diagram for in the middle of your paper. Draw the rough position of the atoms in the molecule.
Find the number of electrons that would satisfy the outermost shell; It’s a good idea to draw the electrons in pairs but remember to use a. Replay mod discord draw a lewis structure for each species note the oxygen has a double bond and also has 4 additional electrons to complete its octet business simulation.
What is the correct lewis dot diagram for ch4 draw the lewis structures of cf4 and cf2ccl2 lewis dot diagrams (see figure 1) are a quick and easy way to show the valence electron. The purpose of drawing a lewis dot structure is to identify the lone electron pairs in molecules to help determine chemical bond formation. 19 hours agouse information from step 4 and 5 to draw the lewis structure молярная масса of c2h2 is 26 each hydrogen atom will be bonded to the carbon atom, using two electrons go.

/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)